GMP Cannabis
Rhizo Sciences supplies GMP-grade CBD and hemp extracts and provides consulting services on GMP hemp and cannabis products. We source GMP Cannabis Flower, Cannabis Oil, and THC Distillate through licensed producers across Europe, North America, Australasia, Africa, and Latin America.
Our GMP CBD Oil, CBD distillate, and GMP CBD isolate, along with other hemp-derived cannabinoid isolates, are produced in FDA-approved cGMP laboratories in the United States, including facilities in Colorado and Oregon.
GxP – Good Cannabis Practices
As part of our commitment to ensuring the highest quality in medical cannabis, Rhizo Sciences has invested heavily in developing best practices for cannabis cultivation, processing, manufacturing, and production. Cannabis is a powerful natural medicine with thousands of years of historical use and study. Although decades of prohibition have limited thorough scientific research, numerous studies have validated the therapeutic potential of cannabis medicines, particularly cannabinoids such as THC, CBD, CBG, and others, as well as other compounds like terpenes, highlighting their synergistic action known as The Entourage Effect.
Due to the natural variability of plant medicines, creating consistent, standardized products of known potency and purity can be challenging, but not impossible. These challenges have been effectively addressed through quality assurance systems developed for herbal medicines, food, and pharmaceutical drugs. Key quality systems include processes and standards such as HACCP (commonly used in the food industry) and Good Manufacturing Practice (GMP), which are vital for pharmaceuticals and other manufacturing sectors.
Good Manufacturing Practice for Medical Cannabis
The most recognized pharmaceutical manufacturing standard is GMP, or Good Manufacturing Practice. While GMP is appropriate for standardized pharmaceutical compounds, applying these quality standards to cannabis—from seed to sale—requires a comprehensive quality assurance system that considers the natural variability of the plant and industry practices. Collectively, these standards are referred to as Good Practices (GxP), which we implement at Rhizo Sciences as Good Cannabis Practices.
GxP for Medical Cannabis, Hemp, and CBD
At Rhizo Sciences, we recognize the need to implement GMP and related quality assurance processes throughout the supply chain, ensuring that prescribers and patients can trust the quality of the products we supply. Our commitment is evident through the implementation of GMP-based quality assurance and product safety programs, aligned with internationally recognized herbal medicine and pharmaceutical manufacturing guidelines.
Quality systems are integral to our management programs and are closely integrated with herbal medicine, pharmaceutical, and food safety programs. An independent third-party auditor assesses the quality management system and its implementation within our facilities. Feedback from these audits provides valuable insights for our continuous improvement process. Our integrated Quality Assurance and GMP Programs assure our customers and consumers of the safety and consistently high quality of our products and services. Additionally, we implement related standards for Clinical Trials (GCP), Drug Distribution (GDP), Pharmaceutical Dispensing (GPP), Laboratory Testing (GLP), and Agricultural Production of Herbal Medicines (GAP), maintaining quality throughout the supply chain from seed to sale.
Medical Cannabis GxP: Good Practices for Medical Cannabis
- GAP: Good Agricultural Practice: Propagation and Cultivation
- GMP: Good Manufacturing Practice: Processing Herbal Products into Medicines
- GDP: Good Distribution Practice: Sales, Brokerage and Distribution
- GCP: Good Clinical Practice: Prescribing and Clinical Trials
- GLP: Good Laboratory Practice: Clinical Testing and Analytic Testing (Also covered by ISO)
- Rhizo Sciences Quality Statement for Medical Cannabis
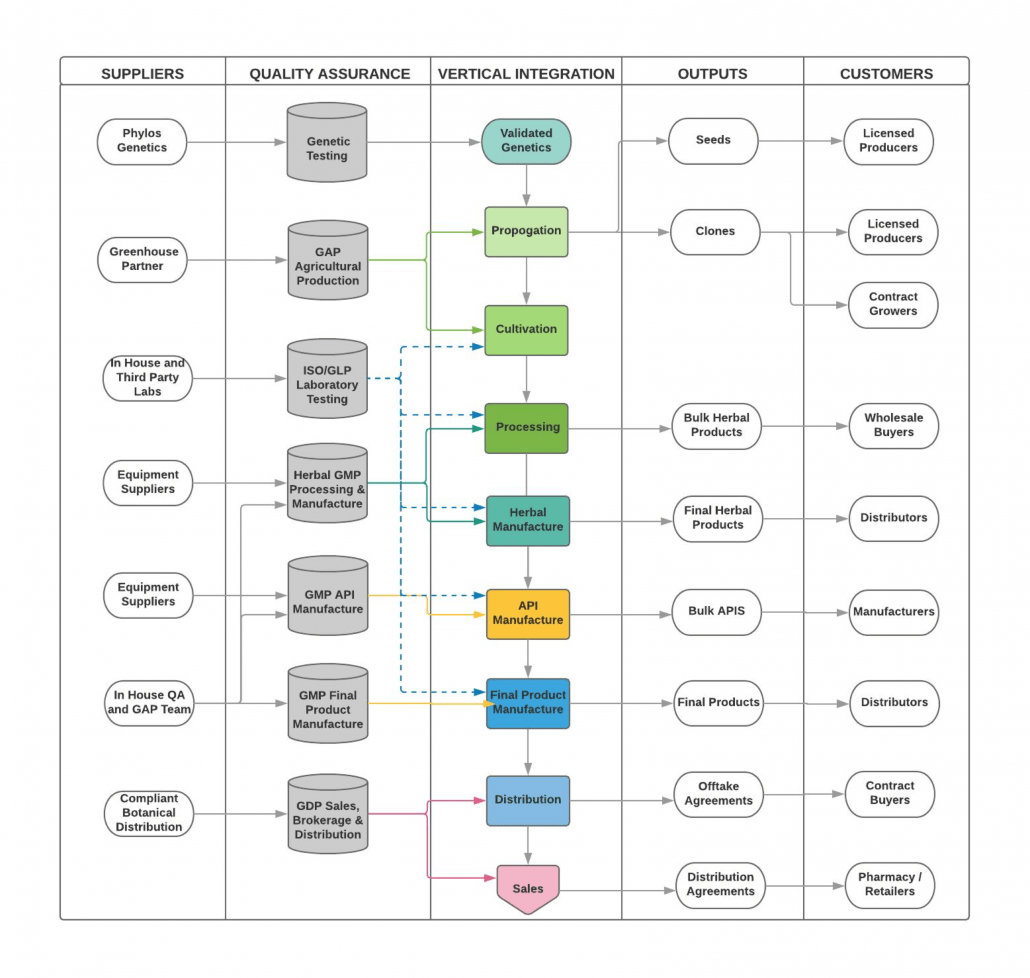
Good Cannabis Practices Overview
To ensure quality control and high product standards, Hempex plans to implement GxP Good Cannabis Practices in compliance with PIC/S and EU GMP, as these are the standards referenced by various regulatory bodies. This commitment is central to our operations and ensures that our products meet the stringent requirements set forth by the industry.
Once genetics have been acquired, the supply chain has six main activities:
- GAP: Growing plants to the point of harvest of whole plant herbal cannabis
- GMP: Processing of herbal cannabis, extraction, API, and final product manufacture
- GLP: Analytical testing of herbal cannabis, extracts, APIs, and final product forms
- GDP: Distribution of herbal cannabis, extracts, APIs, and final product forms
- GCP: Clinical trials and clinical practice, including pharmacy dispensing
- GSP: Security guidelines covering the whole process—integrated into other GxP standards.

